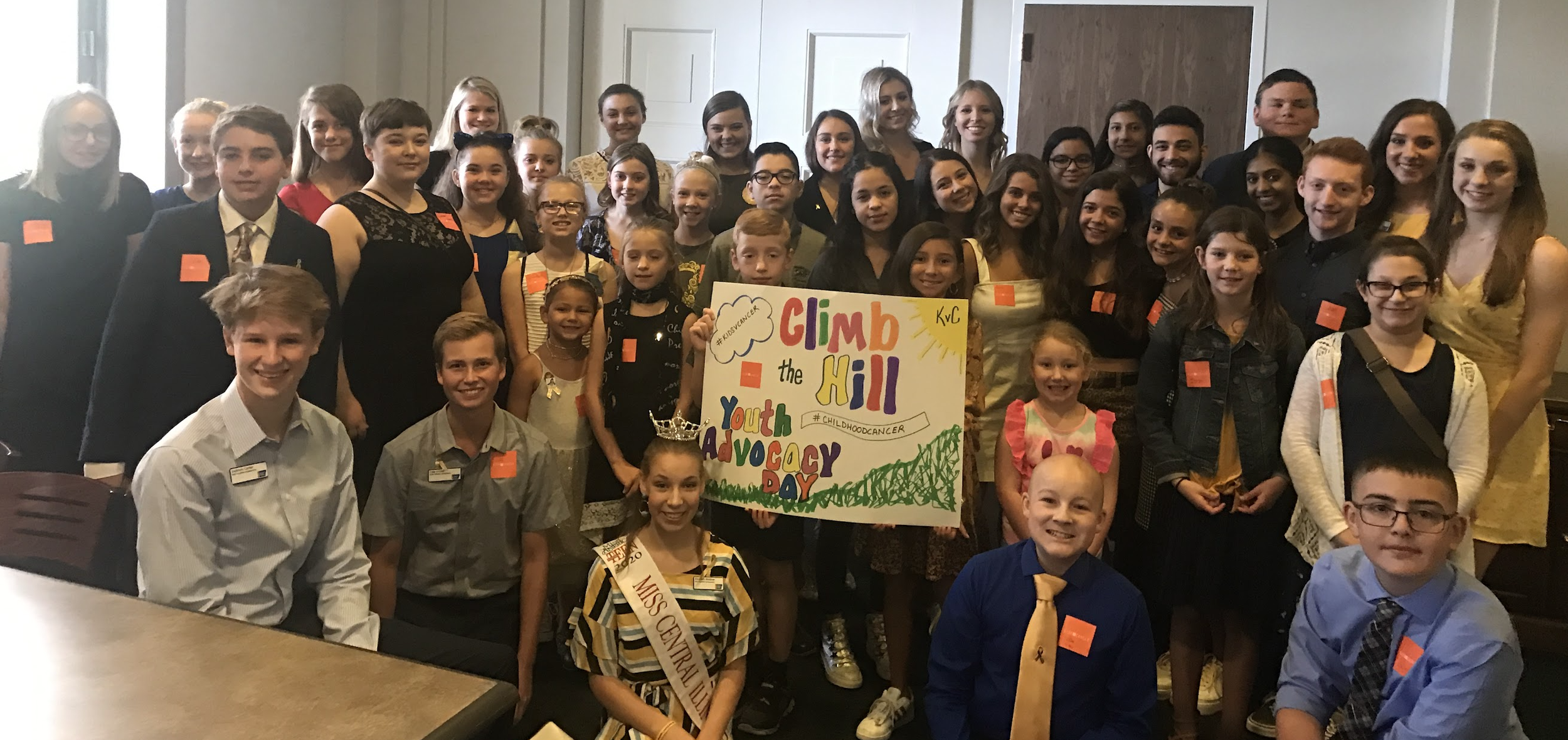KIDS V CANCER
What if 1% of biopharma R&D for cancer drugs was for kids?

Our work
We ask Congress to pass again:
CREATING HOPE REAUTHORIZATION ACT
HR 7384 · first passed in 2012
(21 USC 360ff) · reauthorized in 2016, 2020
Because kids with cancer and other rare, life threatening illnesses need their own drugs:
The Creating Hope Act rare pediatric priority review voucher program provides an incentive for drug development expressly for kids
Problem: The Creating Hope Act sunsets September 31, 2024
Solution: The Creating Hope Reauthorization Act. Since passage of the Creating Hope Act in 2012, over 60 new drugs have been approved for kids with cancer and other rare diseases. Vouchers sell for around $100M.
*no cost to taxpayers or patients
We thank Congress for:
RACE FOR CHILDREN ACT
passed in 2017 (21 USC 355c)
Because single new adult cancer drugs may help kids with cancer:
The RACE for Children Act provides for single drug studies of new adult cancer drugs for kids with cancer
Problem: Only a handful of new cancer drugs were studied in children.
Solution: The RACE for Children Act. Since passage of the RACE for Children Act, over 85% of all new cancer drugs that are appropriate to study in children have planned pediatric studies.
*no cost to taxpayers or patients
We ask Congress to pass:
GIVE KIDS A CHANCE ACT
S 2897/ HR 3433
Because combinations of new adult cancer drugs may cure kids with cancer:
The Give Kids a Chance Act extends the RACE Act to provide for studies of combinations of new adult cancer drugs for kids with cancer
Problem: Single cancer drugs are rarely curative. Cures for cancer require combinations of drugs.
Solution: The Give Kids a Chance Act provides for combinations of new cancer drugs for kids.
* no cost to taxpayers or patients

Leave your parents and teachers behind
Be the voice of childhood cancer
Change the world
Climb the Hill

Advocacy groups endorse Give Kids a Chance Act
Kids v Cancer, Arms Wide Open, Bear Necessities, cc-TDI, ChadTough Defeat DIPG Foundation, Christina Renna Foundation Inc, Clinton County Progressives, Curing Kids Cancer, DADA2 Foundation, DanceBlue Dragon Master Foundation, Elaine Roberts Foundation, End Kids Cancer, EVAN Foundation, Gabriella’s Smile Foundation, Give Kids The World, Gold In September, Gold Rush Cure Foundation, Grandparents In Action, Hannah’s Cans For Cancer, Hunter’s Cancer Journey, Joey’s Wings Foundation, Kick Childhood Cancer Guild, Live Like John, Love Smiles, Lymphedema Advocacy Group, Mackey Family Childrens Cancer Foundation, Making Headway Foundation, Matthew's Hope 4 Miracles, Maynard Childhood Cancer Foundation, McKenna Claire Foundation, Musella Foundation For Brain Tumor Research & Information, Mystic Force Foundation, Oncology Nursing Society, Oral Health Nursing Education and Practice (OHNEP), Our Amazing Fighters, Pediatric Assessment, Learning & Support, Pediatric Brain Tumor Foundation, Pediatric Cancer Foundation, Phoenix Stone Foundation, PNOC Foundation, Press On Fund, Rally Foundation for Childhood Cancer Research, Rett's Roost, Robby Butlers Super Hero Fund, Sadie Keller Foundation, Shepherd Foundation, Swifty Foundation, Syngap1 Foundation, Team G Childhood Cancer Foundation, Teen Cancer America, The Catherine Elizabeth Blair Memorial Foundation, The Cure Starts Now Foundation, The Gold Hope Project, The Kortney Rose Foundation, The Lilabean Foundation for Pediatric Brain Cancer Research, The Maeve McNicholas Memorial Foundation, The Max Cure Foundation, The Scott Carter Foundation, The Taylor Matthews Foundation, The Valerie Fund, Truth 365, Unravel Pediatric Cancer, Until 20, West Virginia Kids Cancer Crusaders, William's Warriors, Wyatt's Warriors, Wylie's Day Foundation, Yusef’s Legacy

Hospitals endorse Give Kids a Chance Act
Children’s Hospital Los Angeles
Children’s Hospital Colorado
Duke University Medical Center
Georgetown University Medical Center
Kentucky Children's Hospital Hematology / Oncology
MD Anderson Cancer Center
Memorial Sloan Kettering Cancer Center
Dana Farber Cancer Institute
Moffitt Cancer Center
St Jude Children’s Research Hospital
Seattle Children’s Hospital
Texas Children’s Hospital
University of Florida Health

Pediatric cancer research consortiums endorse Give Kids a Chance Act
Children's Oncology Group (COG)
Pediatric Brain Tumor Consortium (PBTC)
New Approaches to Neuroblastoma Therapy (NANT)
Pacific Pediatric Neuro-Oncology Consortium (PNOC)
Pediatric Oncology Experimental Investigators' Consortium (POETIC)
Collaborative Network for Neuro-Oncology Clinical Trials (CONNECT)
Beat Childhood Cancer
DIPG Preclinical Consortium